Influenza virus characterization - Summary Europe, February 2023
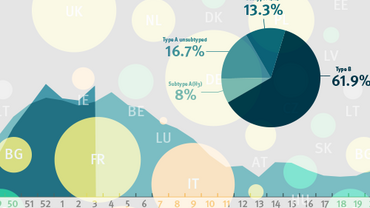
The December 2022 characterisation report, was the third report for the 2022-2023 influenza season. Currently, as of week 08/2023, 231 015 detections have been reported. This represents a 5.2-fold increase in detections compared to the same period of the 2021-2022 season, despite a modest decrease (4%) in the number of samples tested. Of the 2022-2023 detections, 84% were type A viruses, with A(H3N2) and A(H1N1)pdm09 viruses being detected in equal proportions overall, following a rise in the proportion of A(H1N1)pdm09 viruses detected in recent weeks. Since December, the proportion of detections being type B viruses has risen from 6% to 16% and all 3 371 viruses (9% of the total) ascribed to a lineage have been B/Victoria. The epidemic threshold (10% influenza positivity within sentinel specimens) was crossed in week 45/2022 and has remained above this to week 08/2023.
Executive summary
Forty shipments from countries within the WHO European Region were received at the London WHO Collaborating Centre, the Francis Crick Worldwide Influenza Centre (WIC) since the December report. This report focuses on viruses with collection dates after 31 August 2022 for which HA gene sequences were submitted to, and released in, the EpiFluTM database of the Global Initiative on Sharing All Influenza Data (GISAID) in January and February 2023, together with sequences and antigenic data generated at the WIC.
Globally, the great majority of A(H1N1)pdm09 viruses detected in the first 21 weeks of the 2022-2023 season have HA genes in clade 6B.1A.5a.2 (5a.2). Compared to the same period of the 2021-2022 season, as a proportion of type A viruses detected in the WHO European Region, there has been an increase from 7% to 50%. Circulating clade 5a.2 viruses are well recognised by post-infection ferret antisera raised against A/Victoria/2570/2019-like (5a.2) viruses, in vaccines for the northern hemisphere 2022-2023 influenza season, but are recognised less well by post- vaccination sera from humans. As recently circulating viruses carry HA1 K54Q, A186T, Q189E, E224A, R259K and K308R amino acid substitutions compared to A/Victoria/2570/2019, the recommendation was to change the vaccine component to an A/Sydney/5/2021-like (5a.2a) virus for the southern hemisphere 2023 season. Following emergence and geographic spread of viruses with additional HA1 amino acid substitutions of P137S, K142R, D260E and T277A, A/Victoria/4897/2022-like (5a.2a.1) viruses were recommended for use in the 2023-2024 northern hemisphere season.
In Europe and across the world A(H3N2) viruses have been dominant with the great majority of recently detected viruses, as assessed from sequence deposition in GISAID’s EpiFluTM database, falling in the ‘Bangladesh-like’ (3C.2a1b.2a.2 (2)) HA clade. Viruses with clade 2 HA genes have shown extensive genetic drift with some associated antigenic drift which has necessitated development of a new clade nomenclature system (see main text). While post-infection ferret antisera have shown some viruses to induce clade-specific responses, there are various levels of cross-clade reactivity in HI assays. Sera from humans vaccinated with A/Darwin/9/2021-like (2a) viruses has shown significant cross-clade reactivity and has been retained as the vaccine virus recommendation for both 2013 southern hemisphere and 2023-2024 northern hemisphere influenza seasons.
Across the world generally, few B/Victoria-lineage viruses have been detected during first 21 weeks of the 2022- 2023 influenza season though numbers of detections have increased in recent weeks in the WHO European Region. The vast majority of viruses with collection dates in 2023, for which sequences have been deposited in GISAID’s EpiFluTM database, have HA genes that fall in the V1A.3a.2 subgroup with defining HA1 A127T, P144L and K203R amino acid substitutions. Post-infection ferret antisera raised against V1A.3a.2 viruses recognise circulating viruses well, despite the emergence of viruses with a variety of HA1 amino acid substitutions. B/Austria/1359417/2021-like (V1A.3a.2) viruses have been retained as the vaccine virus recommendation for both 2023 southern hemisphere and 2023-2024 northern hemisphere influenza seasons.
No cases of infection with circulating B/Yamagata-lineage viruses have been confirmed since March of 2020. All HA gene sequences from the 77 viruses detected in 2020, inclusive of 16 from the WHO European Region, belonged to genetic clade Y3 and had three HA1 amino acid substitutions (L172Q, D229N and M251V) compared to B/Phuket/3073/2013-like viruses which are still recommended for use in quadrivalent influenza vaccines. There is need to share all B/Yamagata-lineage viruses detected recently for detailed characterisation to determine if there are any in circulation that are not related to Live Attenuated Influenza Vaccines.
Download