Questions and answers on COVID-19: Vaccines
Please note that the information provided on this page was valid as of 20 December 2022.
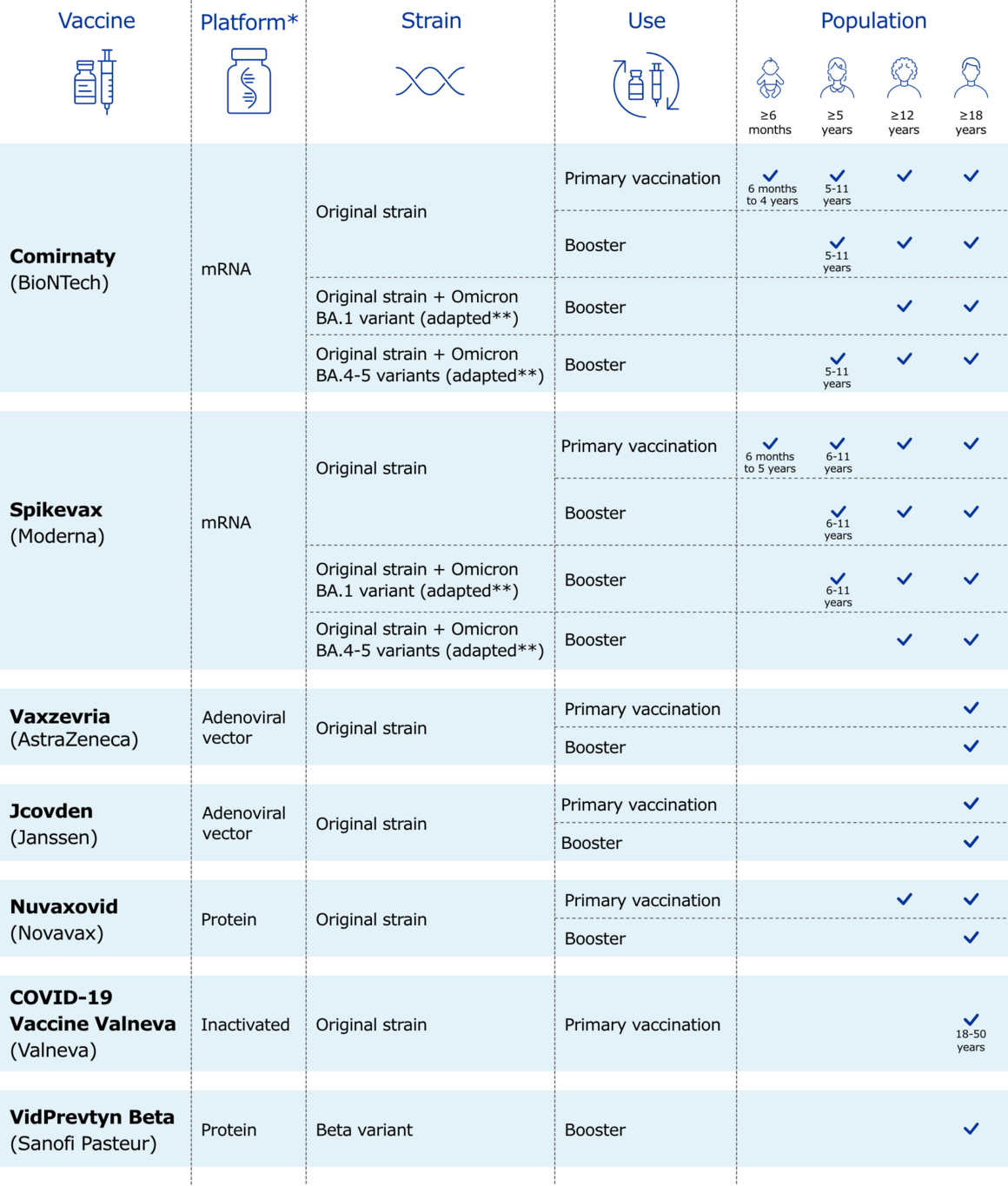
1. Which COVID-19 vaccines are available in the EU?
The following COVID-19 vaccines have been authorised for use in the EU:
- Comirnaty (by BioNTech and Pfizer)
- Spikevax (by Moderna Biotech Spain SL)
- Vaxzevria (by AstraZeneca AB)
- Jcovden (by Janssen-Cilag International NV)
- Nuvaxovid (by Novavax CZ, a.s.).
- COVID-19 Vaccine (inactivated, adjuvanted) Valneva (Valneva Austria GmbH)
- VidPrevtyn Beta (by Sanofi Pasteur)
Most of these vaccines are based on the original strain of the SARS-CoV-2 virus, while VidPrevtyn Beta is based on the Beta variant.
The following adapted versions of COVID-19 vaccines have been authorised for use in the EU. These are updated versions of existing vaccines designed to better match the latest SARS-CoV-2 virus variants in circulation:
- Comirnaty Original/Omicron BA.1
- Comirnaty Original/Omicron BA.4-5
- Spikevax bivalent Original/Omicron BA.1
- Spikevax bivalent Original/Omicron BA.4-5
They are referred to as bivalent as they target the original SARS-CoV-2 virus strain and specific Omicron sub-variants.
2. Are COVID-19 vaccines safe?
COVID-19 vaccines are safe. More than 930 million doses have been administered in the EU/EEA as of 27 October 2022. COVID-19 vaccines are developed following the same legal requirements for quality, safety, and efficacy as for all other vaccines.
Like all vaccines, the effects of COVID-19 vaccines are first tested in the laboratory, including in animals, and then in human volunteers. The European Medicines Agency (EMA) evaluates COVID-19 vaccines against the same high standards as for all other vaccines before they are released for use. More information on the development of COVID-19 vaccines is available in the European Vaccination Information Portal: COVID-19 vaccines (vaccination-info.eu)
Once the vaccines are in use, national authorities and EMA continually monitor their use for any side effects that may occur in people who have received the vaccine.
As with any medicine, some people may experience side effects from a vaccine, but these are usually mild and short-lived. As for all vaccines, close medical supervision is important upon administration of the vaccine.
3. Are COVID-19 vaccines effective?
COVID-19 vaccines authorised for use in the EU/EEA have been very effective at preventing severe disease, hospitalisation, and death, including for infections caused by the more recent Omicron variant. Unvaccinated people remain at a much higher risk of falling severely ill with COVID-19, compared to people who have been vaccinated.
People who have been fully vaccinated and have received a booster are also less likely to pass the virus to others compared with unvaccinated people.
However, the original vaccines are less effective against Omicron infection and against symptomatic disease compared to their effectiveness against the previous variants of the virus. In addition, protection wanes over time.
It is important to get vaccinated with the primary vaccination series to prevent severe disease. Giving an additional or booster COVID-19 vaccine dose to eligible age and risk groups is important to ensure higher and more sustained levels of protection.
Further booster doses are given to populations at a higher risk of severe disease, as protection may wane.
The effectiveness of COVID-19 vaccines is continuously monitored to detect if they are less effective against circulating variants.
As the virus SARS-CoV-2 continues to evolve, the original vaccines are being adapted to ensure optimal protection against COVID-19. Variant-adapted vaccines started to be authorised in September 2022 for use in the EU as boosters. In December 2022 EMA’s Emergency Task Force concluded that adapted mRNA bivalent vaccines targeting the original strain and Omicron BA.4-5 subvariants may be used for primary vaccination.
The adapted vaccines expand the immunity against variants of concern, especially Omicron and related sub-lineages.
This is an area of evolving evidence, and vaccine recommendations or vaccination strategies may need to be adjusted accordingly. Information on the strategies implemented by Member States, is available in the Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA (europa.eu)
4. What are ECDC’s recommendations regarding COVID-19 vaccination?
With the continued high circulation of SARS-CoV-2 in EU/EEA countries, ECDC strongly encourages eligible people who still have not had their primary vaccination series against COVID-19 or any booster dose, to do so as recommended in their countries.
In preparation for the autumn and winter season in 2022, ECDC has also highlighted the importance of a second booster dose for populations most at risk for severe disease.
In a joint ECDC-EMA statement (issued on 6 September 2022) concerning the use and administration of COVID-19 vaccine boosters, it was considered for boosters to be directed as a priority to people who are more at risk of severe disease. Risk factors include
- older age (e.g. above 60 years)
- immune system problems
- underlying medical conditions
- pregnancy.
Priority should be given to residents and staff in long-term care facilities and healthcare workers.
National authorities in the EU make final decisions on the roll-out of vaccines, including booster doses and type of vaccines. For this, countries consider factors such as the spread of infection, the impact of COVID-19 in different populations, and the emergence of new variants.
5.Can vaccinated individuals still get COVID-19 disease?
People who are vaccinated can still become infected with SARS-CoV-2 and infect others, although this occurs less often than in people who are unvaccinated. This is referred to as a ‘breakthrough infection’.
Vaccinated people who still catch the virus are far less likely to fall severely ill, to be admitted to hospital, or to die than unvaccinated people.
Protection conferred by vaccination can decrease over time. The original vaccines are also less effective against the Omicron variant.
Given the risk of infection, vaccinated individuals should continue to follow national recommendations on public health measures to reduce transmission of the virus, such as appropriate hand hygiene, respiratory etiquette, adequate ventilation and the use of face masks where required. Measures to limit the transmission of SARS-CoV-2 are especially important in settings with people at high risk of severe disease and hospitalisation, such as in long-term care facilities.
6. Will COVID-19 vaccines stop the pandemic?
It is likely that the virus that causes COVID-19 will continue to circulate and evolve. It is not possible to predict how infectious or severe new variants of the virus will be. It is therefore very important to achieve and maintain high vaccination coverage. Vaccination remains a key component of the multi-layered approach needed to reduce the impact of SARS-CoV-2.
More efforts are needed to ensure more people get fully vaccinated and receive booster doses, to increase levels of protection and reduce the spread of SARS-CoV-2. This is especially important for those at highest risk of severe disease and particularly in the context of highly transmissible variants like Omicron.
In the meantime, all measures as per national recommendations for controlling the spread of the virus - such as physical distancing, appropriate hand hygiene, respiratory etiquette, adequate ventilation and the use of face masks where required - are still important.
7. Can COVID-19 vaccines protect people against new variants?
Some genetic variants of the virus could potentially reduce effectiveness of the existing vaccines. Scientists around the world are carefully monitoring mutations of SARS-CoV-2 virus to assess how well the currently available COVID-19 vaccines can protect against them.
To date, studies show that the original COVID-19 vaccines initially authorised in the EU/EEA continue to be highly protective against COVID-19-related severe disease, hospitalisation, and death, including during circulation of the highly transmissible Omicron variant. However, vaccines are less effective against Omicron compared to against previous variants.
Adapted vaccines started to be authorised for use in the EU in September 2022, as boosters . In December 2022 EMA’s Emergency Task Force concluded that adapted mRNA bivalent vaccines targeting the original strain and Omicron BA.4-5 subvariants may be used for primary vaccination.
The adapted vaccines are based on the original strain of the virus and the newer Omicron sub-variants, and therefore are also referred to as bivalent vaccines. They add protection against those variants of concern.
8. Is COVID-19 vaccination still necessary, even after getting infected with the virus and recovering?
People who have recovered from a prior infection are less likely to become infected with SARS-CoV-2 and have severe outcomes from COVID-19 (hospitalisation, ICU admission, and death), when compared with people who have not been infected. However, vaccination enhances protection.
Studies show that reinfections with SARS-CoV-2 occur even in people who have had COVID-19. Furthermore, the Omicron variant has led to more reinfections among recovered people when compared with the previously circulating Delta variant.
Evidence is growing that vaccination after infection strengthens protection and further reduces the risk of reinfection. Therefore, COVID-19 vaccination is generally recommended for the eligible population, including those who have recovered from the disease.
9. How is COVID-19 vaccination progressing in the EU/EEA?
The COVID-19 Vaccine Tracker provides information on the uptake of first and second doses of COVID-19 vaccines, as well as additional or booster doses, at the national level and by age, other target groups, and vaccine products, for EU/EEA countries.
10. Are combined or so-called ‘mixed’ vaccination schedules using two different vaccine products safe and effective?
Combined (also referred to as ‘heterologous’ or ‘mixed’) COVID-19 vaccination schedules involve using one type of vaccine for the first dose and a different type of vaccine for the second dose. These mixed schedules are generally well tolerated and may even provide better protection against COVID-19.
Furthermore, the available evidence indicates that using the same vaccine for the primary vaccination course and then a different vaccine for a booster dose appears as good as or better than using the same vaccine for all doses, in terms of immune response.
In particular, receiving an mRNA vaccine for the booster dose when the primary vaccination series was with a viral vector vaccine can improve protection.
Mixed schedules are not new in immunisation and have been used in the past for vaccines against other diseases. Several EU/EEA countries are currently using different combinations of mixed vaccine schedules. Current evidence indicates that these approaches are safe and create a satisfactory immune response.
11. Is an additional or booster dose of COVID-19 vaccine necessary?
Based on current evidence, all eligible individuals who are fully vaccinated (i.e. those who have completed a primary vaccination course) should also get their first booster dose. This is particularly important given the high transmissibility of the Omicron variant and as protection provided by vaccination can decrease over time.
Populations most at risk for severe disease should also get a second booster dose, in preparation for the autumn and winter season in 2022.
Countries are administering a second booster dose to specific populations. Recommendations vary by country, and address mainly older population groups, residents and staff in long-term care facilities, as well as individuals with underlying medical conditions.
Additional doses after the primary vaccination course should also be administered to people with severely weakened immune systems, according to national recommendations. In these instances, additional doses are not considered ‘booster doses’, but an extension of the primary vaccination course, as these people may not achieve adequate protection from the primary course.
Booster doses are given to fully vaccinated people to restore protection after it has partially waned. EMA assesses the data on additional and booster doses to consider whether updates to the product information are appropriate.
According to the currently available evidence, booster doses increase protection, particularly against severe disease.
12. Should adolescents and/or younger age groups be vaccinated against COVID-19?
Children and adolescents who have underlying conditions that put them at higher risk of severe COVID-19 should be considered a priority group for vaccination against COVID-19, as in other age groups. This is to ensure they are protected against severe disease and hospitalisation.
Children and adolescents with no known risk factors are also susceptible to severe disease and hospitalisation, even though COVID-19 infections in these age groups are generally mild and hospitalisation rates remain at much lower levels than in adults.
Vaccination in children and adolescents also reduces the risk of serious complications, such as multi-inflammatory syndrome in children (MIS-C). MIS-C is a rare condition that can cause:
- cardiovascular symptoms
- persistent fever
- inflammation
- gastrointestinal manifestations
The decision of whether to include younger age groups in the COVID-19 vaccination programme is taken at the national level. More information on the strategies of the countries regarding vaccination of younger age groups is available in the Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA (europa.eu).
As of 20 October 2022, the use of the COVID-19 vaccines Comirnaty and Spikevax is authorised in the EU/EEA for use in children aged six months and older.



